
Amnio-Disc™ 500 Micron
Use for Macular Surgery
+919849193447
Key benefits of Amnio-Patch- include:
Anti-inflammatory properties: Helps reduce swelling and discomfort.
Scar tissue prevention: Supports tissue remodeling for smoother healing.
Immune privilege: Lowers the risk of adverse immune responses.
Enhanced tensile strength: Provides durability and flexibility for various applications.
Gamma Sterile

Amnio-Disc™ processed by dehydrated human placenta membrane allograft to be used as biological ocular bandage.
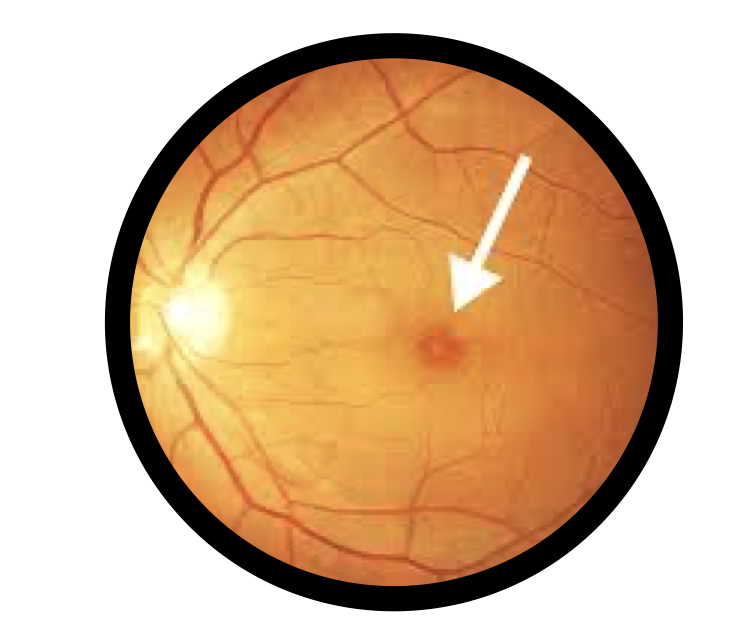
Amnio-Disc™ is a pioneering solution in vitreoretinal surgery, specifically designed for macular hole repair and retinal regeneration. Its advanced formulation leverages the biological properties of amniotic membranes, providing an optimal scaffold for tissue healing and cellular proliferation.
Macular Hole Surgery: Supports retinal tissue repair, aiding in closure and recovery post-surgery.
Persistent Retinal Defects: Acts as a regenerative matrix to facilitate healing in cases where traditional methods may not suffice.
Retinal Detachment Management: Contributes to enhanced wound healing, complementing standard surgical techniques.
Ocular Surface Regeneration: A potential adjunct in innovative ophthalmic procedures, improving surgical outcomes.
Amnio-Disc is CDSCO-approved and ISO 13485-certified, ensuring safety, efficacy, and reliability in advanced ophthalmic applications. Its recognition across multiple countries underscores its effectiveness in transforming retinal care.
Ophthalmic Applications of Amnio-Disc™: The Amnio-Disc is utilized in ophthalmology for various corneal and ocular surface conditions, including:
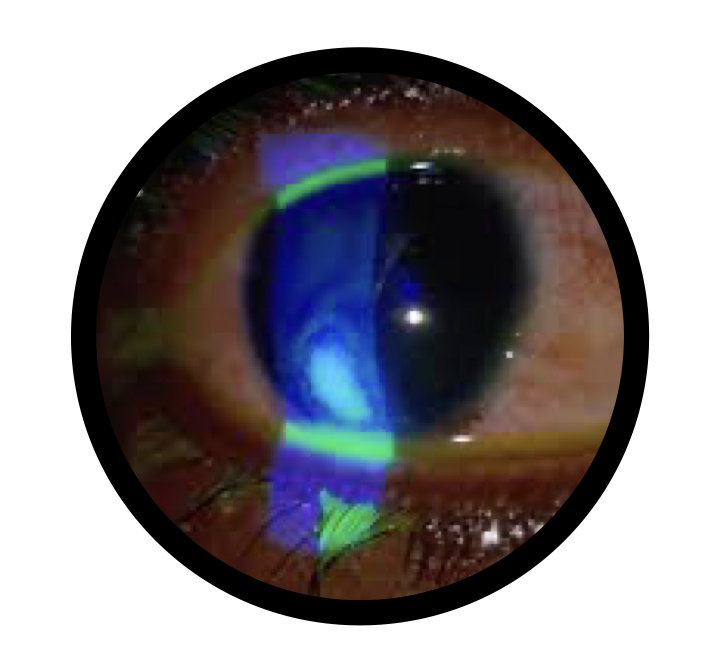
Post-Photorefractive Keratectomy (PRK): Supports epithelial healing and reduces inflammation after laser vision correction.
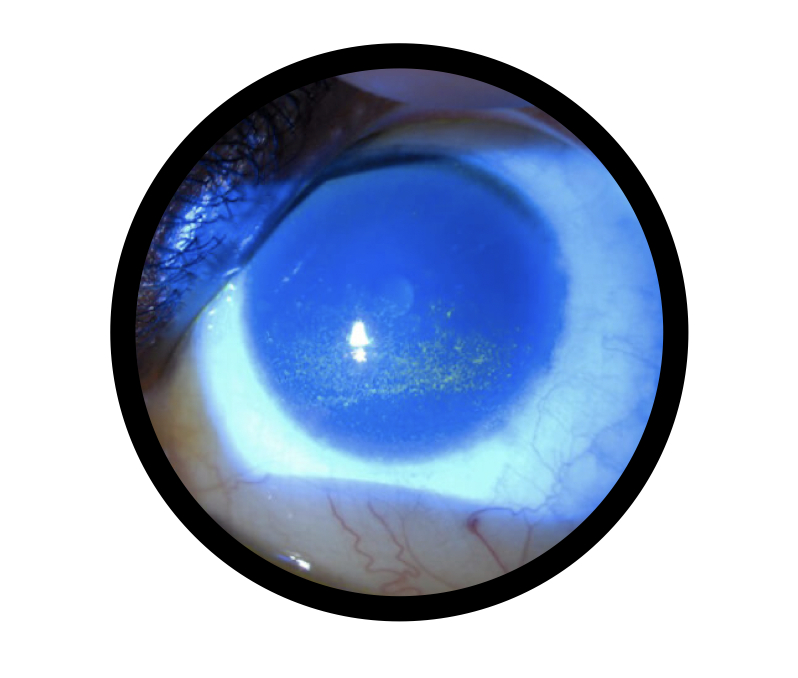
Non-Healing Corneal Ulcers: Provides essential growth factors and structural support to aid in epithelial regeneration.
Dry Eye Disease: Helps restore tear film stability and enhances corneal surface health.
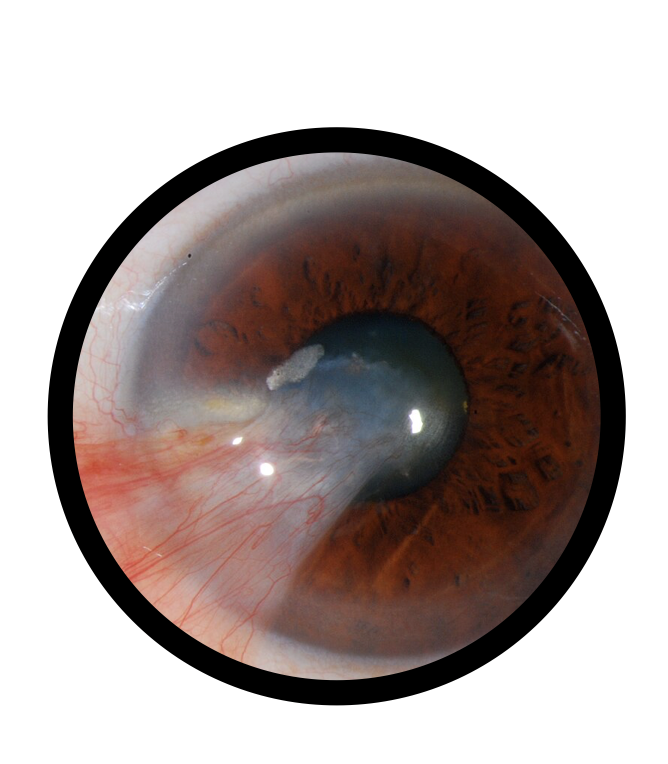
Recurrent Epithelial Defects: Assists in reducing the frequency of epithelial breakdown and accelerating tissue repair.
Concretions: Facilitates healing of damaged ocular surfaces affected by small calcified deposits.
Persistent Epithelial Defect (PED) Over the Cornea: Promotes re-epithelialization in cases of delayed wound healing.
Packing Details : single unit of Amnio-Disc™
Sterilised By : Gamma Radiation.
Single Use
Indication, contraIndications & Application guide, Please refer Package Insert ( Instruction for use)
Storage : Store in a clean and dry environment as ambient room temperature.

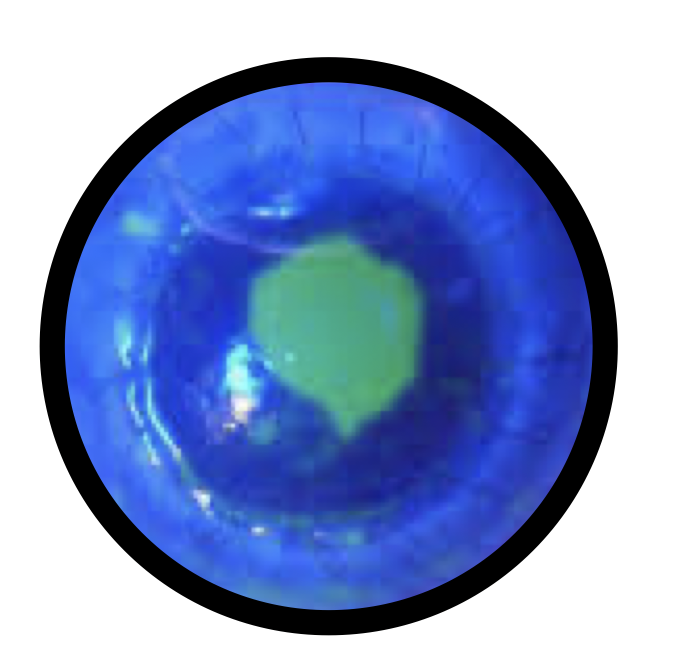
Use for Macular Surgery

Use for Macular Surgery.

Use for Macular Surgery

Use for Macular Surgery.

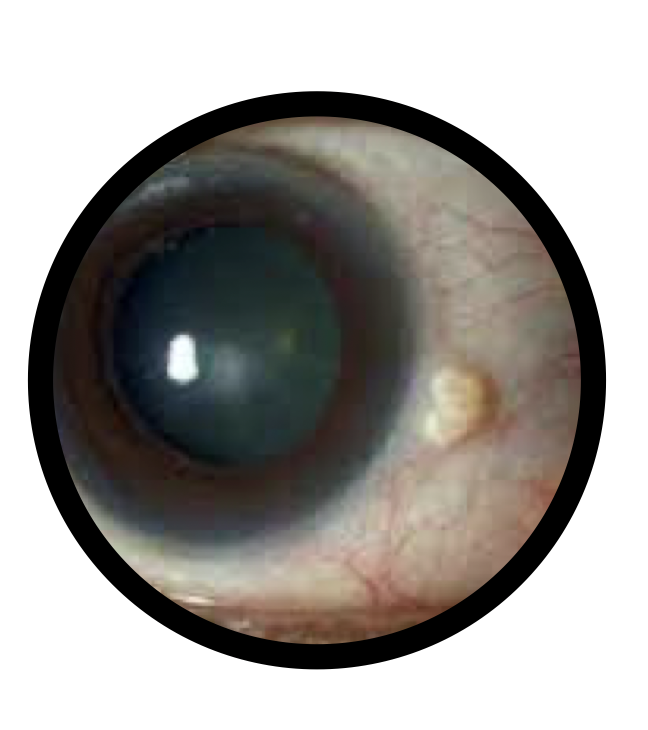
Use for Non healing wound in cornea, non Healing ulcer etc

Use for Non healing wound in cornea, non Healing ulcer etc

Use for Non healing wound in cornea, non Healing ulcer etc

Use for covering the wound over conjctival surface






Amnio-Disc™ extends its regenerative potential beyond macular surgery, offering significant benefits for dry eye disease and non-healing ocular ulcers. Its biological properties help reduce inflammation, enhance epithelial regeneration, and provide a protective environment for tissue recovery.
Acts as a biological scaffold, supporting the restoration of damaged ocular surface cells.
Reduces inflammation and discomfort, aiding in relief from chronic dry eye symptoms.
Enhances tear film stability, promoting long-term ocular surface health.
Delivers anti-inflammatory and anti-scarring effects, accelerating tissue regeneration.
Provides a biocompatible structure, promoting epithelial healing where conventional treatments may be insufficient.
Protects the ocular surface, minimizing further tissue degradation and enhancing recovery.
Macular Hole Surgery: Supports retinal tissue repair, aiding in closure and recovery post-surgery.
Persistent Retinal Defects: Acts as a regenerative matrix to facilitate healing in cases where traditional methods may not suffice.
Retinal Detachment Management: Contributes to enhanced wound healing, complementing standard surgical techniques.
Ocular Surface Regeneration: A potential adjunct in innovative ophthalmic procedures, improving surgical outcomes.
ISO 10993 Biocompatibility Testing
Sterilization Validation
Lot to Lot Sterility Testing
Viral Inactivation Validation
Robust In vitro Comparative Characterization including Residual DNA Quantification, Biomechanical (uniaxial, stuture pullout, ball burst), SEM, Histological, Immunohistochemical, SDS Page, cell culture analysis & Degradation Analysis

Amnio-Disc is derived from amniotic tissue, leveraging its natural regenerative properties for effective wound healing.
It acts as a biological barrier, reduces inflammation, minimizes scar tissue and enhances tissue regeneration.
It is suitable for various wound types including surgical, chronic, and traumatic eye and corneal wounds.
Yes, it is a biocompatible product derived from sterilized human amniotic tissue, ensuring safety.
Use the Amnio-Disc™ as directed by your healthcare professional. Duration may vary based on individual cases.
You can contact us over email on sales@amnio-patch.com